Calculate S° at 25°C for the reduction of PbO(s) , 2PbO(s) + C(s) 2Pb(s) + CO2(g) given these absolute entropies: 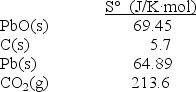
Definitions:
Anti-Communist Credentials
The reputation or record of a person or government for being strongly opposed to communism and its ideologies.
Right-Wing Republicans
Members of the Republican Party in the United States who hold conservative or traditional viewpoints, focusing on issues like national security, fiscal conservatism, and limited government.
Running Mate
The candidate for the vice-presidential position on a presidential ticket, selected by the presidential candidate, often to balance the ticket with respect to ideology, geographic appeal, or demographic characteristics.
Checkers Speech
A televised address by Richard Nixon in 1952 where he refuted accusations of financial improprieties using a personal anecdote involving his dog, Checkers.
Q10: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q29: Calculate the molar solubility of lead (II)fluoride
Q34: At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q58: It is possible for the following
Q64: Calculate K<sub>c</sub> for the reaction 2HI(g)<sub> </sub><sub>
Q94: For the reaction Ni<sup>2+</sup>(aq)+ 2Fe<sup>2+</sup>(aq) <span
Q104: The following reaction is nonspontaneous at
Q109: How many moles of H<sub>2</sub> are produced<sub>
Q115: Which of the following is consistent
Q138: How many minutes would be required to