Find the temperature at which the reaction N2O4(g) 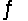 2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm.
2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm. 
Definitions:
Work-in-Process
Inventory items that are in the production process but are not yet completed products.
Parts
Components or pieces that can be combined or assembled to build or repair something.
Raw Materials
Basic materials used in the production process, transformed into finished goods through manufacturing.
Captive Finance Company
A subsidiary whose purpose is to provide financing to customers of a parent company, facilitating sales of the parent company's products.
Q17: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)
Q21: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="Balance the
Q29: At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q35: The reaction 2SO<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="The reaction
Q59: Arrange the following substances in the
Q75: Calculate the percent ionization of formic acid
Q85: Calculate the pH of a 6.7 ×
Q104: Consider the following decay series: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q133: What type of nuclear process is illustrated
Q175: The pH of coffee is approximately 5.0.How