Determine the equilibrium constant (Keq) at 25°C for the reaction Cl2(g) + 2Br- (aq) 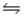 NEWLINE
NEWLINE
2Cl- (aq) + Br2(l)
Definitions:
Statistical Analyses
involve the collection, presentation, and interpretation of data to discover patterns and trends.
Satisfaction
The feeling of pleasure or fulfillment that comes from meeting one's needs, desires, or expectations.
Panic
A sudden, overwhelming fear that produces irrational behavior, hastily made decisions, or the flight response, often in response to a perceived threat or danger.
Anticipation Stage
The phase in project or personal development where preparation and expectations for future events or changes are made.
Q5: The energy equivalent of a mass defect
Q24: In order for a gas to be
Q44: Write the chemical formula of triammineaquodichlorochromium(III)chloride.
Q52: The electron configuration of a Ti atom
Q58: The K<sub>sp</sub> for silver(I)phosphate is 1.8 ×
Q71: An environmental chemist obtained a 200.mL sample
Q85: The total number of electrons in the
Q89: The solubility product for barium sulfate is
Q100: The half-reaction that should occur at
Q109: Choose the substance with the higher entropy