Determine the equilibrium constant (Keq) at 25°C for the reaction Cl2(g) + 2Br- (aq) 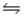 NEWLINE
NEWLINE
2Cl- (aq) + Br2(l)
Definitions:
Negative Symptoms
Features often associated with schizophrenia, including lack of motivation, reduction in spontaneous speech, and diminished emotional expression.
Dopamine
A neurotransmitter involved in controlling movement and posture, modulating mood, and playing a central role in positive reinforcement and dependency.
Typical Drugs
Medications or substances commonly used or prescribed, based on a standard set of medical conditions.
Schizophrenia Treatment
Various approaches, including medication, psychotherapy, and support services, aimed at managing symptoms and improving the quality of life for individuals with schizophrenia.
Q40: The mineral cryolite, Na<sub>3</sub>AlF<sub>6</sub>, is used in
Q40: Write the chemical equation for the reaction
Q42: A 5.2 L sample of a 1.1
Q67: Compare nuclear reactions with chemical reactions in
Q82: Write the formula for the conjugate base
Q87: Which of the following is consistent
Q113: How many coulombs of charge are required
Q137: What is the pH of a 0.30
Q139: Will a 0.1 M solution of NH<sub>4</sub>CN(aq)be
Q148: Complete and balance the following redox