Determine the equilibrium constant for the following reaction at 25°C.
2I- (aq)+ Br2(l) 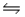
I2(s)+ 2Br-(aq).
Definitions:
Abnormal Return
The difference between the actual return of a security and the expected return based on risk and market performance.
Fundamental Analysts
Professionals who evaluate securities by measuring the intrinsic value of a stock, company, or market, based on financial and economic factors.
Dividend Prospects
The potential for future dividend payments from investments, often assessed to evaluate the attractiveness of stocks.
Future Interest Rates
Expected rates of interest in the future, affecting everything from loans to investments.
Q5: Which of the following processes would
Q12: The only stable isotope of aluminum is
Q33: Based on the following options, select the
Q34: The average person breathes about 20 m<sup>3</sup>
Q43: The molar solubility of manganese(II)carbonate is 4.2
Q78: Calculate the equilibrium constant K<sub>c</sub> for the
Q80: How would you expect the molecule 1,10-phenanthroline
Q87: For the electrochemical cell Ni(s)| Ni<sup>2+</sup>(1 M)||
Q95: Calculate the cell voltage for the
Q109: How many moles of H<sub>2</sub> are produced<sub>