Calculate the Energy Change for the Reaction K(g)+ Br(g) K+(g)+ Br- (G)
Given the Following Ionization Energy (IE)and Electron
Calculate the energy change for the reaction K(g) + Br(g) K+(g) + Br- (g)
Given the following ionization energy (IE) and electron affinity (EA) values 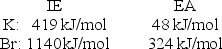
Definitions:
Kerosene
A flammable hydrocarbon liquid commonly used as a fuel in heating, jet engines, and lighting, extracted from petroleum.
Jet Fuel
A type of aviation fuel designed for use in aircraft powered by gas-turbine engines.
Industrial Chemicals
Chemical compounds produced in large quantities for use in various industries, ranging from manufacturing to agriculture.
Crude Oil Refining
The industrial process of transforming crude oil into useful petroleum products such as fuel, gasoline, and diesel.
Q10: How many grams of water are needed
Q18: The vapor pressure of ethanol is 400
Q36: Calculate the density of CO<sub>2</sub>(g)at 100°C and
Q39: Use the Born-Haber cycle to calculate
Q52: For silicon atoms, which ionization energy will
Q67: Of the species NO<sub>2</sub>, NO, and N<sub>2</sub>,
Q68: The sulfide ion, S<sup>2-</sup>,<sup> </sup> is isoelectronic
Q69: Which of the following is the electron
Q81: Indicate the type of hybrid orbitals used
Q97: The term "proof" is defined as twice