The isomerization of cyclopropane to form propene is a first-order reaction. 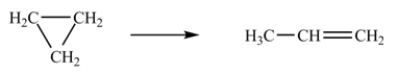 At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
Definitions:
Single-Celled
Referring to organisms that consist of only one cell, carrying out all the functions necessary for life.
Dinoflagellates
A phylum of microorganisms found in marine and freshwater environments, known for their ability to conduct photosynthesis and for some species, to produce bioluminescence and harmful algal blooms.
Alveolates
A group of protists characterized by the presence of alveoli, small sacs underneath their cell membranes.
Kelps
Large brown seaweeds that grow in underwater forests in shallow oceans, known for their high growth rate and the complex habitat they provide for marine life.
Q5: How much enthalpy is necessary to heat
Q9: Over what range of pH is a
Q21: Calculate the pH of a solution that
Q36: The data below were determined for the
Q53: For the first-order reaction 2N<sub>2</sub>O<sub>5</sub> <font face="symbol"></font>
Q58: Arrange the acids H<sub>2</sub>Se, H<sub>2</sub>Te, and H<sub>2</sub>S
Q88: What is the pH at the equivalence
Q103: The solubility of a solid always increases
Q104: Calculate the energy change for the
Q119: Identify the dominant (strongest)type of intermolecular force