Calculate Kp for the reaction 2NOCl(g) 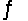 2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 * 10-2.
2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 * 10-2.
Definitions:
Follicle-Stimulating Hormone
A hormone involved in the reproductive processes of both males and females, stimulating the growth of ovarian follicles in females and spermatogenesis in males.
Oxytocin
A hormone and neurotransmitter involved in childbirth and lactation, also associated with behaviors like bonding, trust, and empathy.
Fight or Flight
A physiological response to perceived harmful events, attacks, or threats to survival, preparing the body for defensive or evasive action.
Epinephrine
Also known as adrenaline, a hormone and a neurotransmitter that increases heart rate, muscle strength, blood pressure, and sugar metabolism.
Q2: Which of the following substances is/are bent?
Q19: In how many grams of water should
Q23: Osmium tetroxide, OsO<sub>4</sub>, is a soft crystal
Q33: What is the mole fraction of sodium
Q35: Write the chemical formula for nitric acid.
Q51: To 1.00 L of a 0.100 M
Q72: Which of the following gases would have
Q76: Oxygen gas makes up 21 % of
Q83: Methyl red is a common acid-base indicator.
Q99: Explain the following, on the basis of