The brown gas NO2 and the colorless gas N2O4 exist in equilibrium, 2NO2 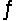 N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.
N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.
Definitions:
Stage IV Pressure Ulcer
A severe injury to the skin and underlying tissue, characterized by extensive tissue damage and possibly involving muscle or bone, typically due to prolonged pressure.
Nursing Diagnosis
A clinical judgment about individual, family, or community experiences/responses to actual or potential health problems/life processes, forming the basis for selecting nursing interventions.
Impaired Skin Integrity
A condition where the skin is damaged, leading to a breakdown in the barrier it provides against infection and injury.
Dressing
A protective or therapeutic covering applied to a wound or surgical site to aid in healing or prevent further injury or infection.
Q2: Given the following compound and its boiling
Q10: An unusual atmospheric reaction leading to
Q14: According to the VSEPR theory, the molecular
Q14: Which one of the following indoor air
Q36: Which of these acids is stronger, H<sub>3</sub>PO<sub>4</sub>
Q38: Without reference to a table, arrange
Q48: Of the pair of compounds given, which
Q75: In which of these gas-phase equilibria is
Q84: How does the entropy change when a
Q122: The bond angles in SF<sub>5</sub><sup>+</sup> are expected