The equilibrium between carbon dioxide gas and carbonic acid is very important in biology and environmental science.
CO2(aq)+ H2O(l) 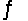
H2CO3(aq)
Based on the equilibrium constant reported for this reaction (Kc = 1.70 * 10-3), there will be more carbon dioxide in solution than carbonic acid.
Definitions:
Conservation
The practice of preserving natural resources and the environment for future generations.
Egocentrism
A cognitive trait where an individual has difficulty understanding or assuming any perspective other than their own.
Lower Socioeconomic Status
A classification for individuals or families with lower income, education, and occupational prestige compared to the societal average, often associated with greater exposure to health, educational, and social challenges.
Head Start
A U.S. initiative that delivers extensive services in early childhood education, health, nutrition, and engages parents, targeting children and their families with low income.
Q9: Arrange the acids HOBr, HBrO<sub>3</sub>, and HBrO<sub>2</sub>
Q10: The heat of vaporization of water
Q11: When comparing acid strength of binary acids
Q20: Complete and balance the following redox
Q64: Use the table of data shown below
Q75: A saturated sodium carbonate solution at 0°C
Q82: The brown gas NO<sub>2</sub> and the colorless
Q107: Calculate the amount of heat needed
Q127: Calculate the pH of a carbonated beverage
Q163: If the pH of stomach acid is