If one starts with pure NO2(g) at a pressure of 0.500 atm, the total pressure inside the reaction vessel when 2NO2(g) 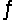 2NO(g) + O2(g) reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
2NO(g) + O2(g) reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
Definitions:
Discount Method
The discount method is a way of understanding the value of money received in the future as being less valuable than money held today, often used in calculating the present value of future cash flows.
Actual Interest Rate
The actual interest rate received or incurred on a loan or investment, considering the impact of compounding.
Discount Rate
The rate of interest imposed on loans obtained by commercial banks and other depository institutions from the Federal Reserve's discount window.
Actual Interest Rate
The real rate of interest earned or paid on an investment, loan, or other financial product, taking into account the effects of compounding.
Q6: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q15: List three primary pollutants removed from automobile
Q37: Ibuprofen is used as an analgesic for
Q39: Describe how to prepare 500.mL of a
Q40: The following reaction is nonspontaneous at
Q45: Calculate the percent ionization of formic acid
Q63: Indicate all the types of intermolecular forces
Q75: A saturated sodium carbonate solution at 0°C
Q76: HI has a normal boiling point
Q78: An organic compound was prepared and purified