Consider the following equilibrium,
4NH3(g)+ 3O2(g) 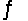
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant when the temperature is increased.
Definitions:
Loanable Funds
The market where savers supply funds for loans to borrowers, influenced by the interest rate.
Tax Rebate
A financial refund provided by the government to taxpayers, typically to stimulate the economy or encourage certain behaviors.
Open-Economy Macroeconomic Model
A framework for understanding and analyzing the interactions between a country's economy and the rest of the world, including trade and capital flows.
Political Events
Occurrences within the political sphere that can influence economic conditions, public sentiment, and governmental policy.
Q18: For the reaction HCONH<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q30: For the reaction X<sub>2</sub> + Y +
Q41: What phase exists at the point labeled
Q43: Aspirin, C<sub>9</sub>H<sub>8</sub>O<sub>4</sub>, slowly decomposes at room temperature
Q45: Given the following data, calculate the boiling
Q61: The reaction 2NO(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="
Q71: Which of these acids is stronger, H<sub>3</sub>AsO<sub>3</sub>
Q82: Which species will have the greatest absolute
Q107: Write an equation showing the net reaction
Q117: Which of the following would be expected