At 250ºC, the equilibrium constant Kp for the reaction PCl5(g) 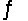 PCl3(g) + Cl2(g) is 1.80.Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC.Calculate the pressure of PCl5 after the system has reached equilibrium.
PCl3(g) + Cl2(g) is 1.80.Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC.Calculate the pressure of PCl5 after the system has reached equilibrium.
Definitions:
Fixed Rates
Interest rates that remain constant over the lifetime of a financial instrument, unaffected by market fluctuations.
Floating Interest
An interest rate that changes over the life of a loan or mortgage, based on the current market conditions or an index.
Solvency
The ability of an entity to meet its long-term debts and financial obligations.
Times Interest Earned Ratio
A financial ratio that measures a company's ability to meet its debt obligations by comparing its income before interest and taxes to its interest expenses.
Q13: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q15: Which of the following liquids would have
Q23: Calculate the molality of a solution containing
Q43: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q51: HCN is classified as a weak acid
Q66: The reaction C<sub>4</sub>H<sub>10</sub> <font face="symbol"></font> C<sub>2</sub>H<sub>6</sub> +
Q79: Polyethylene plastic consists of long chains of
Q89: Determine the pH of a KOH solution
Q92: The data below refer to the following
Q139: Which one of the following substances will