True/False
In the reaction HNO3 + NH3 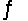 NH4+ + NO3-, NH4+ and NH3 are a conjugate acid-base pair.
NH4+ + NO3-, NH4+ and NH3 are a conjugate acid-base pair.
Definitions:
Related Questions
Q2: In the Hall process, _ is reduced
Q6: Assuming equal concentrations of conjugate base and
Q28: The rate constant of the reaction
Q31: The normal freezing point of ammonia
Q62: Calculate the cell emf for the
Q67: At 25°C the rate constant for the
Q76: Which one of the following reagents is
Q80: The equilibrium constant expression for the reaction<br>CuO(s)+
Q86: When an aqueous solution of AgNO<sub>3</sub> is
Q102: The molar solubility of tin(II)iodide is 1.28