At 340 K, Kp = 69 for the reaction H2(g) + I2(g) 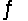 2HI(g) . 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340 K. What is the total pressure inside the cylinder when the system comes to equilibrium?
2HI(g) . 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340 K. What is the total pressure inside the cylinder when the system comes to equilibrium?
Definitions:
Prepaid Expenses
Future expenses that have been paid in advance and are accounted for as assets on a balance sheet until they are incurred.
Accrual Basis
An accounting method that records revenues and expenses when they are earned or incurred, regardless of when the cash is actually exchanged.
Accounts Receivable
Amounts due to a company from its clients for the supply of goods or services, which have yet to be paid for.
Accounts Payable
The amount of money a company owes to its creditors for goods or services purchased on credit.
Q14: Some KCl is dissolved in water 25°C,
Q35: Each of the following substances is a
Q40: According to VSEPR theory, which of the
Q46: A spontaneous endothermic reaction always<br>A)causes the surroundings
Q46: A solution is prepared by mixing 500.mL
Q75: Which of the following aqueous solutions has
Q84: The meniscus for water is curved upward
Q89: At 1500°C the equilibrium constant for
Q98: Appropriate units for a first-order rate constant
Q152: What mass of sodium formate (HCOONa)must be