At 1500°C the equilibrium constant for the reaction CO(g) + 2H2(g) 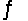 CH3OH(g) has the value Kp = 1.4 10-7. Calculate G° for this reaction at 1500°C.
CH3OH(g) has the value Kp = 1.4 10-7. Calculate G° for this reaction at 1500°C.
Definitions:
Payroll Tax Expense
Payroll Tax Expense represents the taxes that an employer is liable to pay based on the wages and salaries of employees, including social security, Medicare, and unemployment taxes.
Employer's Payroll Taxes
Taxes that employers are responsible for paying on behalf of their employees, including Social Security, Medicare, and unemployment taxes.
Payroll Register
A detailed document that records the earnings, deductions, and net pay for all employees for each pay period.
Gross Payroll
The total amount of money paid by an employer to its employees before deductions such as taxes, retirement contributions, and health insurance.
Q1: Complete and balance the following redox
Q3: The element oxygen was prepared by
Q5: Write the chemical formula of the oxide
Q18: Complete and balance the following redox
Q23: The half-reaction that should occur at
Q40: Find the emf of the cell described
Q41: The regions of the earth's atmosphere listed
Q80: Give the coordination number (C.N.)and oxidation number
Q117: Which one of the following reactions
Q153: The oxides SO<sub>2</sub> and N<sub>2</sub>O<sub>5</sub> will form