For the equilibrium reaction 2SO2(g) + O2(g) 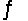 2SO3(g) , Hºrxn = -198 kJ/mol. Which one of these factors would cause the equilibrium constant to increase?
2SO3(g) , Hºrxn = -198 kJ/mol. Which one of these factors would cause the equilibrium constant to increase?
Definitions:
Place Cells
Neurons in the hippocampus that become active when an animal is in a particular location, playing a significant role in navigation and spatial memory.
Hippocampus
A structure in the brain's temporal lobe involved in memory formation, spatial navigation, and the processing of emotional responses.
Korsakoff Syndrome
A chronic memory disorder often associated with excessive alcohol consumption, characterized by amnesia, confabulation, and impaired executive function.
Alcohol Dependency
A medical condition characterized by a strong desire to consume alcohol despite harmful consequences, leading to physical and psychological dependence.
Q4: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)+ 2296
Q17: Which one of the following would slow
Q34: The K<sub>sp</sub> value for lead(II)chloride is 2.4
Q39: A negative sign for <span
Q64: Aluminum forms a layer of aluminum
Q71: During osmosis<br>A)pure solvent diffuses through a membrane
Q84: In which of the following would the
Q96: The activation energy for the reaction CH<sub>3</sub>CO
Q105: Krypton has a higher melting point than
Q108: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many