Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J 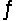
CO(g)+ H2(g).What will happen to the concentration of carbon monoxide if the temperature of this system is raised?
Definitions:
Forward Contract
A non-standardized agreement between two parties to buy or sell an asset at a specified future time at a price agreed upon today.
Spot Rates
The current cost at which one can buy or sell a certain currency, ready for instant delivery.
Merchandise
Items or products that a company buys to sell to its customers in the course of its business.
Journal Entries
The foundational records in accounting that detail all financial transactions, using debits and credits to maintain balance.
Q5: How much enthalpy is necessary to heat
Q49: Indicate all the types of intermolecular forces
Q50: Calculate the percent ionization of formic acid
Q62: Calculate the approximate freezing point of a
Q72: Determine the mass percent HCl in a
Q72: Two moles of PCl<sub>5</sub> are placed in
Q97: Identify the dominant (strongest)type of intermolecular force
Q105: At 25°C, by what factor is the
Q106: Consider a solution made from a nonvolatile
Q112: Aspartic acid (C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>), one of the 20