The reaction 2NO(g) 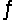 N2(g) + O2(g) is exothermic, Hºrxn = -180 kJ/mol.Which one of these statements is true?
N2(g) + O2(g) is exothermic, Hºrxn = -180 kJ/mol.Which one of these statements is true?
Definitions:
Ruff Degradation
A chemical process used to convert monosaccharides into smaller sugars or alditols.
Optically Active
Refers to a substance that can rotate the plane of polarized light, indicating chiral molecules not superimposable on their mirror image.
Alditol
A type of sugar alcohol formed by reducing the aldehyde or ketone group in aldoses or ketoses, respectively.
Silver Oxide
A chemical compound of silver and oxygen that's used in some batteries and as a mild oxidizing agent in organic synthesis.
Q10: For the common allotropes of carbon
Q25: Which is the correct equilibrium constant expression
Q53: Using the thermodynamic data provided below, calculate
Q56: The normal boiling point of bromine is
Q60: You have 500.0 mL of a buffer
Q64: The pH of coffee is approximately 5.0.
Q98: Automobile radiators usually carry a sticker that
Q122: The bond angles in SF<sub>5</sub><sup>+</sup> are expected
Q127: Arrange the following in order of increasing
Q143: Arrange the acids HOCl, HClO<sub>3</sub>, and HClO<sub>2</sub>