Consider this reaction at equilibrium: 2SO2(g) + O2(g) 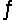
2SO3(g) , Hºrxn = -198 kJ/mol
If the volume of the system is compressed at constant temperature, what change will occur in the position of the equilibrium?
Definitions:
Unspoken Rules
Implicit norms or conventions that are understood and followed by members of a community or society but are not formally documented.
Professional Image
Professional Image is the perception or depiction of an individual or company as competent, reputable, and suitable within a professional context.
Personal Characteristics
Personal characteristics are the traits, qualities, and attributes that define an individual's personality and behavior.
Pre-Employment Testing
The use of assessments and exams by employers to evaluate potential hires' skills, abilities, or personality traits before offering employment.
Q6: Given that the heat of vaporization of
Q10: An unusual atmospheric reaction leading to
Q39: Acid rain is less of a
Q74: The OH<sup>-</sup> concentration in a 7.5 *
Q82: The vapor pressure of water at 20°C
Q83: In the reaction Ag<sup>+</sup>(aq)+ Cl<sup>-</sup>(aq) <span
Q87: Equilibrium is established for the reaction 2X(s)+
Q100: The reaction 2NO<sub>2</sub>(g)<font face="symbol"></font> 2NO(g)+ O<sub>2</sub>(g)is suspected
Q127: Calculate the pH of a carbonated beverage
Q160: In the reaction HSO<sub>4</sub><sup>-</sup>(aq)+ OH<sup>-</sup>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"