The Equilibrium Constants for the Chemical Reaction N2(g)+ O2(g) Hº < 0
B)The Partial Pressure of NO(g)is Less at 2,200 K
The equilibrium constants for the chemical reaction N2(g) + O2(g) 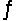
2NO(g) are KP = 1.1 * 10-3 and 3.6 * 10-3 at 2,200 K and 2,500 K, respectively. Which one of these statements is true?
Definitions:
Review
The process of examining something with the possibility of making changes based on the assessment.
Situation
A set of circumstances or a state of affairs at a particular time or in a particular place.
Proposed Solution
A suggested plan or method to solve a problem or address a challenge, often presented in a formal context.
Close
The final part of a communication or document that summarizes the main points or provides a conclusion.
Q18: Plasma is the fluid portion of blood.The
Q20: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,
Q33: Dissolving an ionic solid in water always
Q34: Benzoyl chloride, C<sub>6</sub>H<sub>5</sub>COCl, reacts with water to
Q37: The pH of a sample of river
Q49: For the chemical reaction system described by
Q58: Find the concentration of calcium ions in
Q62: Acetic acid has a heat of fusion
Q65: When the reaction 2O<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="When
Q106: The rate constant of a first-order reaction,