When the reaction 2H2S(g) 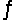 2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g) ?
2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g) ?
Definitions:
Merton
Refers to Robert K. Merton, an influential American sociologist known for his theories on social structure, anomie, and the sociology of science.
Anomie
A condition in society characterized by a breakdown or absence of social norms and values, often leading to a state of normlessness.
Normlessness
A state or condition where social norms are unclear, weak, or not widely followed, often leading to social disorientation.
Social Breakdown
Refers to the deterioration of the social fabric and structures, leading to a decline in societal norms and values, often resulting in chaos or disorder.
Q3: Name four "greenhouse gases" besides carbon dioxide.
Q8: What is the molality of a 0.142
Q29: Write out the steps in the mechanism
Q36: Find the temperature at which the reaction
Q58: Sulfur can be separated from lead
Q63: Which one of the following combinations cannot
Q83: Methyl red is a common acid-base indicator.
Q94: Which of the following substances would have
Q99: Sulfur can be separated from lead
Q107: An aqueous fructose solution having a density