Find the temperature at which the reaction N2O4(g) 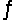 2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm.
2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm. 
Definitions:
Bona Fide Purchaser
A person who buys property in good faith, without notice of any prior claims or disputes over the property, and is therefore protected under law.
Attachment
A legal procedure by which a court of law, at the request of a creditor, designates specific property owned by the debtor to be transferred to the creditor or sold for the benefit of the creditor.
Financing Statement
A document filed to give public notice of a secured party's interest in the debtor's personal property.
Charter Business
A company that operates under a charter, offering specified services or engaging in a particular type of business, often subject to special regulations or privileges.
Q20: Find the nuclear binding enrgy of
Q24: The data below refer to the following
Q33: Name the complex ion [Cr(en)<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub>]<sup>2+</sup>.
Q35: What happens to nitrogen dioxide in sunlight?
Q46: A solution is prepared by mixing 500.mL
Q61: Write a net ionic equation for the
Q86: Peroxodisulfate ion can oxidize iodide ions to
Q86: What is the net ionic equation
Q92: Calculate the pH of a 3.5 *
Q94: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,