If the reaction 2H2S(g) 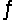 2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.0120. Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g) ?
2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.0120. Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g) ?
Definitions:
Middle-School Child
A young individual, typically between the ages of 11 and 14, attending middle school which bridges elementary and high school.
Tandem Gait
A method of walking where one foot is placed directly in front of the other, testing balance and coordination, often used in neurological examinations.
Caregiver Role Strain
Stress or burden experienced by a person providing care for someone else, often leading to physical or emotional exhaustion.
Nursing Diagnosis
An evaluation regarding the reactions of an individual, family, or community to real or possible health issues or life events.
Q19: The K<sub>sp</sub> of CaF<sub>2</sub> is 4 *
Q30: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q35: Which of the following has the greater
Q42: Use VSEPR theory to predict the molecular
Q81: A first-order reaction has a rate constant
Q84: For the first-order reaction, A <font face="symbol"></font>
Q94: Draw a Lewis structure for PF<sub>5</sub> that
Q95: Which of the following is not true
Q97: A quantity of liquid methanol, CH<sub>3</sub>OH, is
Q120: Complete and balance the following redox