Consider the reaction N2(g)+ 3H2(g) 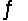
2NH3(g). The production of ammonia is an endothermic reaction.Will heating the equilibrium system increase or decrease the amount of ammonia produced?
Definitions:
Sources
Origins or points of origin from which something comes or can be obtained.
Dose Form
The physical form in which a drug is produced and dispensed, such as tablets, capsules, drops, or injections.
Treat Disease
The process of managing and providing medical care to alleviate or cure a disease.
Licensed Practitioner
A healthcare provider who has obtained the necessary education and skills and is licensed to provide specified healthcare to patients.
Q14: A 50.0 mL sample of 2.0 *
Q16: Calculate the pH of a solution that
Q26: A solution was prepared such that the
Q30: What is the osmotic pressure of a
Q58: Find the concentration of calcium ions in
Q64: Use the table of data shown below
Q74: Using the thermodynamic data provided below, determine
Q95: Consider the chemical reaction 2NH<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q98: Which one of these equations represents
Q103: The solubility of a solid always increases