Two moles of PCl5 are placed in a 5.0 L container.Dissociation takes place according to the equation PCl5(g) 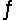
PCl3(g)+ Cl2(g).At equilibrium, 0.40 mol of Cl2 are present.Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
Definitions:
Anticipated Policy
A governmental or organizational policy that is expected to be implemented in the future, often already influencing current behaviors and decisions.
Price Level
A measure of the average prices of goods and services in the economy at a given time.
Output
The total amount of goods and services produced by an economy, a sector, or a company over a specific period of time.
Real Wages
Wages adjusted for inflation, representing the purchasing power of wages in terms of goods and services, unlike nominal wages which are not adjusted for inflation.
Q13: Aluminum forms a layer of aluminum
Q17: Which of the following substances should have
Q28: When the reaction 2H<sub>2</sub>S(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="When
Q29: Write out the steps in the mechanism
Q39: A solution is prepared by adding 40.3
Q73: An example of a covalent network solid
Q83: The equilibrium between carbon dioxide gas and
Q89: Calculate the silver ion concentration in a
Q98: Choose the substance with the higher entropy
Q108: The solubility of a salt increases as