Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J 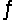
CO(g)+ H2(g).If additional gaseous water is added to this reaction mixture, what will happen to the temperature of the mixture?
Definitions:
Vernix Caseosa
A white creamy substance that covers the skin of the fetus during pregnancy, providing protection.
Fetoscopy
A medical procedure involving the insertion of a fetoscope to visually examine a fetus inside the uterus.
Ultrasound
A medical imaging technique that uses high-frequency sound waves to create images of structures within the body.
Amniocentesis
A medical procedure used in prenatal diagnosis, involving the extraction of a small amount of amniotic fluid surrounding the fetus to study genetic and chromosomal information.
Q7: Based on the phase diagram shown below,
Q26: A rate constant will have the units
Q30: For the reaction X<sub>2</sub> + Y +
Q35: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q44: Calculate the pH of a buffer solution
Q62: What is the pH of a 0.20
Q81: Solid ammonium hydrogen sulfide is introduced into
Q95: The pH of a solution that is
Q103: Indicate all the types of intermolecular forces
Q132: The number of atoms in a face-centered