Short Answer
Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J 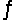
CO(g)+ H2(g).Which way will the reaction shift if the pressure on the system is increased?
Definitions:
Related Questions
Q4: Acid strength decreases in the series: HCl
Q20: For the following reaction at equilibrium,
Q30: What is the osmotic pressure of a
Q33: Which of the following correctly lists species
Q35: Calculate the standard cell emf for the
Q35: How many moles of NaF must be
Q59: Which response includes all of the
Q60: Consider the two gaseous equilibria: <br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q88: What is the concentration of H<sup>+</sup> in
Q89: The graphs below all refer to the