For the following reaction at equilibrium, which choice gives a change that will shift the position of equilibrium to favor formation of more products? 2NOBr(g) 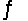
2NO(g) + Br2(g) , Hºrxn = 30 kJ/mol
Definitions:
365-Day Year
A method used in finance that assumes a year has 365 days and is used for calculating interest rates or other financial metrics.
Total Amount
The complete sum of money required or received, often including principal, interest, taxes, and fees.
Ordinary Interest
Interest calculated on the basis of a 360-day year, which is commonly used in calculating interest for loans, mortgages, and bonds.
360-Day Year
An accounting method that simplifies interest calculation by assuming all months have 30 days, resulting in a 360-day year.
Q13: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q20: For the following reaction at equilibrium,
Q25: For the overall chemical reaction shown below,
Q30: For the reaction X<sub>2</sub> + Y +
Q34: The average person breathes about 20 m<sup>3</sup>
Q39: Acid rain is less of a
Q94: Ethanol and acetic acid react to form
Q95: Which one of these net ionic
Q102: Which one of the following substances is
Q109: The pH of a 0.02 M solution