Kc for the reaction CO2(g)+ H2(g) 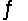
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of hydrogen gas in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Individual Parts
Components or pieces that when combined form a whole system or object.
Physiological Psychology
A branch of psychology that studies the relationship between behavior and the physical processes of the brain and other parts of the nervous system.
University of Leipzig
The University of Leipzig is a renowned research university located in Leipzig, Germany, established in 1409, making it one of the world's oldest universities.
Wilhelm Wundt
A German psychologist widely considered the father of experimental psychology, who established the first psychological laboratory.
Q3: Hydrogen iodide decomposes according to the equation
Q8: The most prevalent noble gas in our
Q14: Which one of the following indoor air
Q22: Substitute natural gas can be synthesized by
Q49: If one starts with pure NO<sub>2</sub>(g)at a
Q67: Which is the formula for the hydronium
Q83: Methyl red is a common acid-base indicator.
Q108: Which of the following properties indicates the
Q137: What is the pH of a 0.014
Q147: What is the pH of a solution