Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq) + HCO3- (aq) 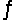 HSO3-(aq) + H2CO3(aq) .
HSO3-(aq) + H2CO3(aq) .
Ka1(H2SO3) = 1 * 10-2; Ka1(H2CO3) = 4.2 * 10-7
Definitions:
Offline Friends
Individuals with whom one maintains a friendship in the physical world outside of digital or online platforms.
Online Discussions
Conversations that occur over the internet in forums, social media platforms, or other digital spaces.
Psychotherapy
A form of therapy involving talking to a mental health professional to address and manage mental health issues.
Psychologist
A professional specializing in diagnosing and treating mental, emotional, and behavioral disorders, often through counseling.
Q26: Which one of the following crystallizes in
Q61: Consider an electrochemical cell constructed from
Q69: For the reaction H<sub>2</sub>O<sub>2</sub>(g) <span class="ql-formula"
Q72: Two moles of PCl<sub>5</sub> are placed in
Q73: The rate law predicted by the following
Q89: Indicate all the types of intermolecular forces
Q102: Write the chemical formula for sulfuric acid.
Q126: Al(OH)<sub>3</sub> is an amphoteric hydroxide. Write a
Q127: Gold can be electrochemically "plated" onto
Q140: What mass of water would need