The equilibrium constant for the reaction C6H5COOH(aq) + CH3COO-(aq) 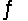 C6H5COO-(aq) + CH3COOH(aq)
C6H5COO-(aq) + CH3COOH(aq)
Is 3.6 at 25°C. If Ka for CH3COOH is 1.8 * 10-5, what is the acid dissociation constant for C6H5COOH?
Definitions:
Allocation Of Risk
The process of defining and assigning the financial or operational responsibilities associated with potential risks in contractual agreements.
Parties
Individuals, groups, or entities that enter into a legal agreement or are involved in a legal proceeding.
Agreement
A mutual understanding or arrangement between two or more parties about their relative rights and responsibilities.
Good Faith Purchaser
An individual who buys property without knowledge of any existing claims or defects against it.
Q7: Based on the phase diagram shown below,
Q31: Write out the steps in the mechanism
Q54: Predict the sign of <span
Q64: The osmotic pressure of a 0.010 M
Q70: An environmental chemist obtained a 200.mL sample
Q76: For the chemical reaction system described by
Q93: For the reaction 2C(graphite)+ H<sub>2</sub>(g) <span
Q94: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,
Q136: Which of these lists of molecules is
Q138: In the following half equation, which