The equilibrium constant for the reaction C7H15COOH(aq) + HCOO-(aq) 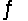 C7H15COO-(aq) + HCOOH(aq)
C7H15COO-(aq) + HCOOH(aq)
Is 7.23 * 10-2 at 25°C. If Ka for formic acid (HCOOH) is 1.77 * 10-4, what is the acid dissociation constant for C7H15COOH?
Definitions:
Health Care Provider
A professional offering services intended to improve an individual's health, including doctors, nurses, and therapists.
Home Visit
A healthcare service or assessment conducted in the patient's residence.
Readmission Rate
The proportion of patients who are readmitted to a hospital or health care facility within a specific time period after discharge, often used as a quality indicator.
Case Management
A collaborative process of assessment, planning, facilitation, and advocacy for options and services to meet an individual's comprehensive health needs.
Q6: The compound CFCl<sub>3</sub> is used as a/an<br>A)enzyme<br>B)anesthetic<br>C)gaseous
Q16: Arrange these reactions according to increasing
Q19: Which one of the following is not
Q23: Ozone in the stratosphere protects animals and
Q28: Given that E<sub>a</sub> for a certain biological
Q29: How many coulombs (C)of electrical charge must
Q70: An environmental chemist obtained a 200.mL sample
Q77: Determine the equilibrium constant K<sub>p</sub> at
Q89: At 1500°C the equilibrium constant for
Q140: What is the pH of a 0.0055