The equilibrium constant for the reaction C6H5COOH(aq) + CH3COO-(aq) 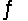 C6H5COO-(aq) + CH3COOH(aq)
C6H5COO-(aq) + CH3COOH(aq)
Is 3.6 at 25°C. If Ka for CH3COOH is 1.8 * 10-5, what is the acid dissociation constant for C6H5COOH?
Definitions:
Cleaner Production
Environmental management strategies and practices focusing on reducing waste and pollutants throughout the production process, promoting more efficient and sustainable industrial operations.
Mandatory Separation
A policy or regulation requiring the division or separation of entities or services, often to prevent conflicts of interest or maintain competition.
Refundable Deposits
Payments made in advance for a service or product that can be returned to the payer upon cancellation of the service or return of the product.
Curbside Charges
Fees imposed for services or goods provided at the curbside, such as waste collection or food delivery.
Q3: What phase exists at the point labeled
Q13: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q19: Which element group includes the most reactive
Q26: The reaction rates of many spontaneous
Q29: At a certain temperature, the data below
Q33: In the Mond process, nickel is purified
Q65: For the electrochemical cell Pt(s)| H<sub>2</sub>(1 atm)|
Q71: Solid sodium iodide is slowly added to
Q90: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q117: Which one of the following reactions