Ammonium chloride solutions are slightly acidic, so they are better solvents than water for insoluble substances such as Ca(OH)2. Identify two reactions that, added together, give the overall reaction below, and determine Kc for the overall reaction.
Ca(OH)2(s)+ 2NH4+(aq) 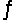 Ca2+(aq)+ 2NH3(aq)+ 2H2O
Ca2+(aq)+ 2NH3(aq)+ 2H2O
Given: Ksp for Ca(OH)2 is 4.0 * 10-8; Kb for NH3 is 1.8 * 10-5.
Definitions:
Scientific Thinking
A method of inquiry that involves observing phenomena, asking questions, forming hypotheses, conducting experiments, and drawing conclusions based on evidence.
Assumptions
Conditions or principles that are taken as true or as certain to happen, without proof, for the purpose of argument or investigation.
Factor Of Production
A resource used in the production process to generate goods and services, such as labor, land, and capital.
Output
The aggregate output of goods or services from a firm, industry, or the economy as a whole.
Q29: What is the concentration of O<sub>2</sub>(g)in water
Q31: The concentration of Mg<sup>2+</sup> in seawater is
Q36: Which of these species is not an
Q41: The reaction A(g)+ 2B(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="The
Q66: What mass of sodium nitrite must be
Q72: Which solution will have the lowest pH?<br>A)0.10
Q84: When 2.0 * 10<sup>-2</sup> mole of nicotinic
Q84: For the first-order reaction, A <font face="symbol"></font>
Q85: Which response gives the products of hydrolysis
Q117: Which one of the following reactions