Calculate the equilibrium constant Kc for the following overall reaction:
AgCl(s)+ 2CN-(aq) 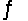 Ag(CN)2-(aq)+ Cl-(aq)
Ag(CN)2-(aq)+ Cl-(aq)
For AgCl, Ksp = 1.6 * 10-10; for Ag(CN)2-, Kf = 1.0 * 1021.
Definitions:
Research And Development (R&D) Center
A facility or department dedicated to investigating and developing new products, processes, or technologies.
Gross Domestic Product (GDP)
The total market value of all goods and services produced within a country in a given period, often used as an indicator of the economic health of a country.
Skills Shortage
A situation where the demand for a specific skill set exceeds the supply of individuals with those skills within a labor market.
Global Talent
Highly skilled professionals who are sought after worldwide for their expertise and ability to contribute significantly to various sectors and industries.
Q2: Draw and label the geometric isomers of
Q16: Write the chemical formula of pyrite.
Q60: What is the coordination number of cobalt
Q72: At a particular temperature the first-order gas-phase
Q85: An electrochemical cell based on the
Q94: For PbCl<sub>2</sub> (K<sub>sp</sub> = 2.4 * 10<sup>-4</sup>),
Q95: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q112: Aspartic acid (C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>), one of the 20
Q129: Which of these acids is the strongest?<br>A)H<sub>2</sub>SO<sub>3</sub><br>B)H<sub>2</sub>SeO<sub>3</sub><br>C)H<sub>2</sub>TeO<sub>3</sub>
Q153: The oxides SO<sub>2</sub> and N<sub>2</sub>O<sub>5</sub> will form