For the reaction SbCl5(g) 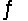 SbCl3(g)+ Cl2(g),
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate G at 800 K and 1 atm pressure (assume S° and H° do not change with temperature).
Definitions:
Reference Line
A line on a drawing that serves as a basis or guide, commonly used to reference dimensions or to anchor annotation symbols such as those for welding.
Leg
A supporting structure or component of an object, typically extending downwards to provide stability or support.
Root Opening
The gap between the parts being joined before welding.
Tail
In engineering drawings, often refers to the part of a callout or notation that provides additional information, such as specifications or notes.
Q6: Under standard-state conditions, which of the
Q28: When the reaction 2H<sub>2</sub>S(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="When
Q31: For the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q33: Dissolving an ionic solid in water always
Q34: Calculate the cell emf for the
Q52: The solubility of strontium carbonate is 0.0011
Q53: Calculate the pH at the equivalence point
Q90: The mass of a nucleus is always
Q100: Which isotope, when bombarded with nitrogen-15, yields
Q118: Will a 0.1 M solution of NH<sub>4</sub>NO<sub>2</sub>(aq)be