Calculate the equilibrium constant Kc for the net reaction shown below.
AgI(s)+ 2NH3(aq) 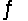 Ag(NH3)2+(aq)+ I-(aq)
Ag(NH3)2+(aq)+ I-(aq)
For AgI, Ksp = 8.3 * 10-17; for Ag(NH3)2+, Kf = 1.5 * 107.
Definitions:
Short-run Phillips Curve
A graphical representation showing the inverse relationship between unemployment rates and inflation rates in the short-term.
Per-capita Income
The average income earned per person in a certain area, calculated by dividing the area's total income by its total population.
Rational Expectations School
An economic idea that assumes individuals make predictions about the future based on all available information and past experiences in a rational manner.
Monetary Policy
The process by which a central bank controls the supply of money in an economy, typically targeting inflation, employment, and economic growth.
Q11: For the following reaction at equilibrium,
Q12: The mineral cryolite, Na<sub>3</sub>AlF<sub>6</sub>, is used in
Q19: What concentration of Ni<sup>2+</sup> ion remains in
Q52: In the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="In the
Q56: Which of these metals will reduce water
Q67: Hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)decomposes according to the
Q70: Consider the [CoCl<sub>6</sub>]<sup>4-</sup> ion. Which response includes
Q81: Consider the following electrochemical cell: U |
Q93: For the reaction 2C(graphite)+ H<sub>2</sub>(g) <span
Q124: Consider the reaction Fe + Sn<sup>2+</sup>(1