At 1500°C the equilibrium constant for the reaction CO(g) + 2H2(g) 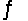 CH3OH(g) has the value Kp = 1.4 10-7. Calculate G° for this reaction at 1500°C.
CH3OH(g) has the value Kp = 1.4 10-7. Calculate G° for this reaction at 1500°C.
Definitions:
Viagra
A pharmaceutical drug, sildenafil citrate, used to treat erectile dysfunction and, in certain cases, hypertension, by increasing blood flow to specific areas of the body.
Depo-Provera
Depo-Provera is a hormonal birth control method administered through injection, providing protection against pregnancy for a period of time.
Sexual Response Cycle
A sequence of physical and emotional phases that occur when an individual becomes sexually aroused and engages in sexually stimulating activities, including phases such as excitement, plateau, orgasm, and resolution.
Blood Pressure
The measurement of the force exerted by circulating blood against the walls of the body's arteries.
Q3: What is the H<sup>+</sup> ion concentration in
Q12: A typical radius of an atomic
Q23: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q27: A possible reaction leading to removal
Q41: Which of the following species is the
Q60: What is the coordination number of cobalt
Q77: The total number of electrons in the
Q93: For the reaction 2C(graphite)+ H<sub>2</sub>(g) <span
Q103: Tritium is a radioisotope of hydrogen
Q121: Write the chemical formula for phosphoric acid.