Consider the reaction CO(g)+ 2H2(g) 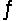 CH3OH(l)at 25°C.
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate value of the equilibrium constant (Kp)for this reaction at 25°C.
Definitions:
Adequacy
Adequacy refers to the suitability of something to meet the needs in its context or the sufficiency in quantity or quality to fulfill a specific requirement.
Consideration
Something of value exchanged between parties within a contract, serving as the reason or motive for entering into the agreement.
Adequacy
The sufficiency to meet the needs or requirements; often assesses whether something is enough in quantity or meets a certain standard.
Consideration
Something of value (such as money, services, or goods) exchanged between parties in a contract.
Q1: Complete and balance the following redox
Q12: Which of these substances is the active
Q62: In transition metal complexes, the metal ions
Q71: The neutral monodentate ligand L forms the
Q71: Calcium carbonate decomposes at high temperatures to
Q92: In the gas phase, formic acid
Q107: Consider the following standard reduction potentials in
Q125: Which element is associated with the term
Q136: Which of these metals will not reduce
Q157: Morphine, C<sub>17</sub>H<sub>19</sub>NO<sub>3</sub>, is often used to control