For the reaction 3H2(g)+ N2(g) 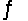 2NH3(g), Kc = 9.0 at 350°C. What is the value of G at this temperature when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
2NH3(g), Kc = 9.0 at 350°C. What is the value of G at this temperature when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
Definitions:
Milgram Experiment
A psychological experiment conducted by Stanley Milgram in the 1960s to study obedience to authority, where participants were instructed to administer electric shocks to another person.
Stanford University Prison Experiment
A psychological study conducted by Philip Zimbardo in 1971 at Stanford University, where students were assigned roles of prisoners and guards to explore the effects of perceived power.
Generalization
Drawing a conclusion about a certain characteristic of a population based on a sample from it.
Logical Support
The provision of reasons or evidence to justify a claim or argument.
Q10: Name the complex ion [CuCl<sub>3</sub>Br(NH<sub>3</sub>)<sub>2</sub>]<sup>2-</sup>.
Q11: Ammonium chloride solutions are slightly acidic, so
Q19: Which one of the following is not
Q45: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q46: Complete and balance the nuclear equation <img
Q48: Write a net ionic equation for the
Q77: A 8.0 M solution of formic acid
Q97: The only stable isotope of aluminum is
Q106: How many minutes would be required
Q110: What current is needed to deposit 0.500