Consider the reaction CO(g)+ 2H2(g) 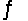 CH3OH(l)at 25°C.
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate S°(H2(g)).
Definitions:
Volume
The amount of space occupied by an object or substance, often measured in liters, cubic meters, or gallons.
Material
Any substance or matter from which something can be made or constructed.
Density
The mass per unit volume of a substance, often measured in grams per cubic centimeter (g/cm^3) or kilograms per cubic meter (kg/m^3).
Volume
The amount of three-dimensional space occupied by an object or enclosed within a container, usually measured in cubic units.
Q8: Write two balanced chemical equations for the
Q12: Which one of the following can function
Q21: Calculate the pH of a solution that
Q24: Phosphoric acid is the most important of
Q43: Acid strength decreases in the series HI
Q54: Write the chemical formula of magnetite.
Q91: The Rb-87/Sr-87 method of dating rocks
Q93: Suppose that ammonia, applied to a field
Q95: Which one of these net ionic
Q104: The equilibrium constant at 427°C for