The equilibrium constant at 427°C for the reaction N2(g) + 3H2(g) 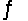 2NH3(g) is Kp = 9.4 10-5. Calculate the value of G° for the reaction under these conditions.
2NH3(g) is Kp = 9.4 10-5. Calculate the value of G° for the reaction under these conditions.
Definitions:
Creditors
Individuals, businesses, or entities to whom money is owed by debtors, typically due to the provision of goods, services, or loans.
Cash Flow
The complete spectrum of financial inflow and outflow in a business, vitally affecting its liquidity.
Stockholders
Individuals or entities that own shares in a corporation, giving them certain rights like voting on corporate affairs.
Tax Rate
The chunk of financial earnings a corporation or individual pays as tax.
Q4: Given the following standard reduction potentials in
Q10: An unusual atmospheric reaction leading to
Q13: Calcium metal is produced by electrolysis of<br>A)CaSO<sub>4</sub><br>B)CaF<sub>2</sub><br>C)CaCO<sub>3</sub><br>D)Ca(OH)<sub>2</sub><br>E)CaCl<sub>2</sub>
Q32: The half-cell reaction for the oxidation
Q55: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="Balance the
Q68: Consider an electrochemical cell based on
Q72: Two moles of PCl<sub>5</sub> are placed in
Q80: Calculate the percent ionization of cyanic acid,
Q100: For which type of titration will the
Q109: Using the thermodynamic data provided below, calculate