At 700 K, the equilibrium constant for the reaction CO(g)+ H2O(g) 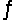 CO2(g)+ H2(g)is 5.10.What is G° for this reaction at this temperature?
CO2(g)+ H2(g)is 5.10.What is G° for this reaction at this temperature?
Definitions:
Psychoanalysis
A therapeutic approach developed by Freud, focusing on unconscious motivations and conflicts as the roots of psychological distress.
Positive Outcome
An event or result that is beneficial, favorable, or desirable.
Inherited Traits
Characteristics or features passed down from parents to offspring through genes.
Statistical Tests
Procedures in statistics used to determine if there is a significant difference between groups or a significant relationship between variables.
Q3: What is the H<sup>+</sup> ion concentration in
Q6: The ion [Co(NH<sub>3</sub>)<sub>6</sub>]<sup>2+</sup> is octahedral and high
Q11: When comparing acid strength of binary acids
Q13: Which of the following cannot be plausibly
Q25: When liquid phosphorus trichloride reacts with water,
Q38: Of the three oxides SiO<sub>2</sub>, MgO, and
Q44: On a neutron number versus proton number
Q48: Write a net ionic equation for the
Q88: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q135: Calculate the pH of a 0.14 M