For the reaction HCONH2(g) 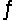 NH3(g) + CO(g) , Kc = 4.84 at 400 K. If H° for this reaction is 29 kJ/mol, find Kc at 500 K.
NH3(g) + CO(g) , Kc = 4.84 at 400 K. If H° for this reaction is 29 kJ/mol, find Kc at 500 K.
Definitions:
Fixed Manufacturing Cost
Expenses that do not change with the level of production, such as rent, salaries, and equipment maintenance.
Units Produced
The total number of completed units of product manufactured during a given period.
Selling Price
The amount of money charged to the customer for a product or service, potentially including costs of production, distribution, and a markup for profit.
Contribution Margin
The gap between sales income and variable expenses, showing the amount that goes towards covering fixed expenses and creating earnings.
Q5: Equilibrium constants are known for the following
Q8: Choose the substance with the higher entropy
Q29: Which of these reactions is an
Q37: The half-reaction that occurs at the
Q40: The dissociation of solid silver chloride in
Q40: Chlorine gas is prepared commercially by<br>A)electrolysis of
Q79: What is the coodination number of silver
Q107: The energy equivalent of 1 amu
Q118: How many coulombs of charge are
Q129: Consider the reaction 2Fe<sup>3+</sup>(aq)+ Fe(s) <img