Equilibrium constants are known for the following reactions:
S(s)+ 3/2O2(g) 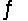
SO3(g)Kc = 9.2 * 1023
SO3(g) 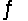
SO2(g)+ 1/2O2(g)Kc = 4.8 *10- 4
Thus, for the reaction S(s)+ O2(g) 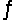
SO2(g), Kc = 4.4 * 1020.
Definitions:
Olfactory Bulb
A structure in the forebrain involved in olfaction, or the sense of smell, where odor information from the nose is processed and sent to the brain.
Concentration
Amount of solute per unit volume of a solution.
Cones
Photosensitive cells located in the retina of the eye, responsible for color vision and the ability to perceive fine details.
Fovea
The fovea is a small depression in the retina of the eye where visual acuity is highest. It is densely packed with cones and is crucial for detailed vision.
Q13: At 400ºC, K<sub>c</sub> = 64 for the
Q26: The reaction rates of many spontaneous
Q34: What is the molarity of a solution
Q35: Calculate the standard cell emf for the
Q44: Identify the conjugate base of HCO<sub>3</sub><sup>-</sup> in
Q65: For the electrochemical cell Pt(s)| H<sub>2</sub>(1 atm)|
Q86: Which one of the following reactions
Q104: In the reaction, 2N<sub>2</sub>O <font face="symbol"></font> 2N<sub>2</sub>
Q108: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q142: Which one of these statements about strong