For the reaction CuS(s)+ H2(g) 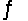 H2S(g)+ Cu(s),
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate G at 798 K and 1 atm pressure (assume S° and H° do not change with temperature).
Definitions:
Power Distance
A cultural dimension that describes the extent to which members of a society accept and expect power to be distributed unequally.
Expertise
Specialized skill or knowledge in a particular field, gained through education, experience, or training.
Uncertainty Avoidance
The degree to which a culture tolerates ambiguity and uncertainty.
Conflict
A situation where two or more parties have incompatible objectives, interests, or needs.
Q3: What is the H<sup>+</sup> ion concentration in
Q12: A standard hydrogen electrode is immersed in
Q13: At 400ºC, K<sub>c</sub> = 64 for the
Q22: Write the chemical formula of the peroxide
Q45: Complete and balance the following redox
Q70: For the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q72: The solubility product for calcium phosphate is
Q76: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q80: Give the coordination number (C.N.)and oxidation number
Q110: Charcoal samples taken from holes dug at