For the reaction CuS(s)+ H2(g) 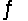 H2S(g)+ Cu(s),
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 798 K and 1 atm pressure.
Definitions:
Medical Model
The view that psychological disorders are medical diseases with a biological origin.
Psychological Disorders
Psychological disorders are patterns of behavioral, cognitive, or emotional symptoms that impact multiple areas of life, leading to distress for the person experiencing these symptoms.
Chemical Imbalance
A hypothesis that mental health conditions are associated with changes or imbalances in brain chemicals or neurotransmitters.
Depressed
A mental health condition characterized by persistent feelings of sadness, hopelessness, and a lack of interest or pleasure in activities.
Q13: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q19: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q21: The pOH of a solution is 10.40.Calculate
Q27: Which one of the following reagents is
Q46: The electron configuration of a Cr<sup>3+</sup> ion
Q48: Describe why addition of a catalyst does
Q68: Identify the conjugate acid-base pairs in the
Q68: Determine the equilibrium constant (K<sub>p</sub>)at 25°C
Q88: What is the concentration of H<sup>+</sup> in
Q127: Gold can be electrochemically "plated" onto