For the reaction SbCl5(g) 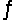 SbCl3(g)+ Cl2(g),
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 800 K and 1 atm pressure.
Definitions:
Bad Debt
A financial loss that occurs when a debtor fails to repay a loan or credit extended, considered non-recoverable and potentially eligible for a tax deduction.
Professional Gambler
Professional Gambler is an individual for whom gambling is considered a trade or business, allowing them to deduct losses and expenses related to their gambling activities as business expenses.
Illegal Information
Information obtained or used in a manner that violates laws or regulations.
Illegal Parking
The act of parking a vehicle in a restricted or unauthorized location, which is against the law and may result in fines.
Q9: Three means used to concentrate ores are
Q12: The reaction 2SO<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="The reaction
Q15: How many grams of nickel would be
Q23: The Hall process involves the reduction of
Q33: Alkali metal hydrides are very reactive with
Q35: Protactinium-234 has a half-life of 1 minute.How
Q65: What terms describe the geometric isomers that
Q100: Which isotope, when bombarded with nitrogen-15, yields
Q107: Consider the following standard reduction potentials in
Q154: Due to a highway accident, 150 L