For the reaction 3H2(g)+ N2(g) 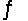 2NH3(g), Kc = 9.0 at 350°C. Calculate G° at 350°C.
2NH3(g), Kc = 9.0 at 350°C. Calculate G° at 350°C.
Definitions:
External Causes
Factors or circumstances outside the control or influence of an individual that contribute to a particular outcome or situation.
Internal Causes
Causes that originate within an individual, such as personal beliefs, emotions, or motives.
Refute
To prove a statement, theory, or argument to be wrong or false.
Stereotype Threat
The risk of confirming negative stereotypes about one's group, which can hamper performance in a variety of settings.
Q1: Which one of these metals would normally
Q8: Predict the number of unpaired electrons in
Q15: An unknown substance was added to a
Q36: According to the band theory, which of
Q87: What is the pH of an aqueous
Q94: Which of the following is an advantage
Q95: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q112: Aspartic acid (C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>), one of the 20
Q115: Calculate the minimum voltage required for
Q144: Which is not a characteristic property of